Vaccine hesitancy or misconception of efficacy?

In India, as of April 5th, 2021, there are 7,91,05,163 vaccine doses administered through 12,31,148 sessions. These include healthcare workers (HCWs), frontline workers (FLWs) and beneficiaries aged more than 45.
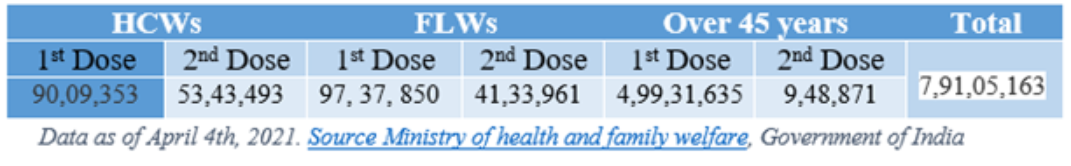
In a nation with an elderly population of nearly 104 million (aged 60 or above), only 79 million are vaccinated, including healthcare and frontline workers in 80 days, is a big concern. These low numbers may stem from various reasons, and reluctance to get vaccinated counts as one of the biggest amongst them.
When COVID-19 hit, it overturned the world and burdened healthcare resources to an unbelievable extent. Even in such an immense challenge, Scientists have managed to perform an incredible feat and developed vaccines rapidly, yet people are hesitant to take the COVID-19 vaccination. People are extremely cautious, have been inquisitive about the vaccines, and have raised questions regarding the efficacy and risks of the vaccines without fully understanding the context and viability of the questions they raise.
Nevertheless, is this a good enough reason why people are hesitant to vaccination? If, yes then we really should spread more awareness regarding what does “efficacy of vaccines” mean.
What does vaccine efficacy mean?
Above all, we should know the difference between efficacy and effectiveness. CDC defines vaccine efficacy as when a study carried out under ideal conditions, for example, during a clinical trial. Whereas, vaccine effectiveness is what when a study carried out under typical field (that is, less than perfectly controlled) conditions. In other words, how well a vaccine works under real-world conditions once people outside of clinical trials receive the vaccine.
Let us take it from the beginning that would be a clinical trial. So when a vaccine is under clinical trial the primary endpoint of the vaccine is to focus on the rising number of incidence, in other words, to prevent new cases. After evaluating the safety and dosage of a vaccine in a phase 1 trial, it would go to a large population to check the efficacy and safety of that particular vaccine and that is phase 3 of a clinical trial.
The results of phase 3 trials would decide the efficacy i.e. the X percentage reduction in the occurrence of disease among the vaccinated group (treatment arm of a trial).
Comparison of COVID-19 vaccines-
A major concern is that people started comparing vaccines without knowing enough about the circumstances under each vaccine trials done. Such as the location and time of the year when the trial is done. Moderna (mRNA-1273) clinical trial was completely done in the US and Pfizer/BioNTech (BNT162b2, mRNA) trial primarily done in the US. These vaccines were under trial during summers between the third quarter (Q3) to quarter fourth (Q4) of the year 2020. However, the Johnsons & Johnsons (J&J) trial primarily done in South Africa and Brazil during the last quarter (Q4) of 2020 to the first quarter (Q1) of 2021and in this Countries virus itself was different. The J&J trial took place as variants of COVID-19 emerged and became dominant infections in these Countries. These variants were somehow responsible to made study participants sick, which could be a foremost reason behind the low efficacy of the J&J vaccine. In South Africa, most of the cases in the J&J trial were that of the variant despite that the vaccine still significantly reduce the infection.
COVID-19 Vaccines and their efficacy percentage
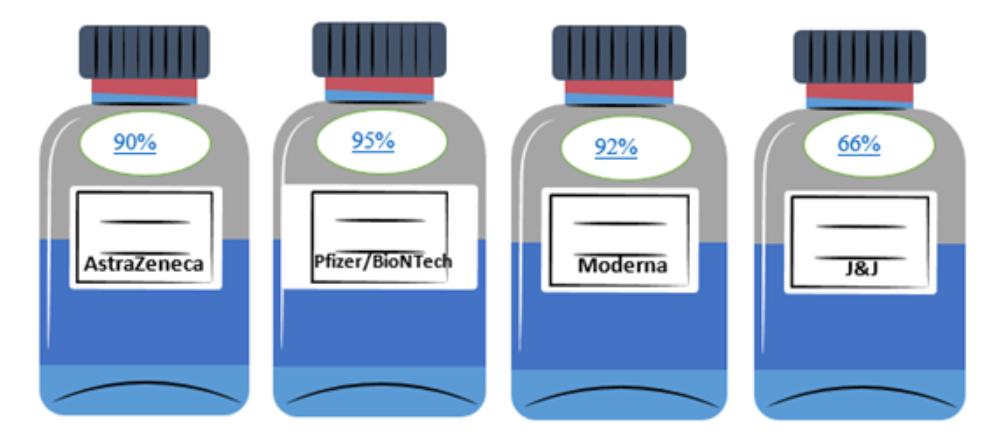
Above is the efficacy of different vaccines in which the maximum efficacy reported by Pfizer/BioNtech with 95% and the minimum is Johnsons & Johnsons (J&J) with 66%. This percentage states that there are 95 and 66 per cent reduction in the number of incidences i.e. new cases of the disease in the vaccines group (treatment group) compared with the placebo group (Control group). A vaccine with an efficacy of 95% does not necessarily mean it is better than other vaccines. Similarly, a vaccine with the efficacy of 66% does not mean that 44% of people who receive that vaccine will contract the virus.
The goal of a COVID-19 vaccine program is not necessary to get to COVID zero, but it is to detain this virus to remove its ability to get a serious disease, hospitalisations and death.
This is a historic pandemic. If we recall the tragic days of 2020 when millions of people lost their lives, and medical professionals were working hard to accommodate patients, hospitals were running out of beds and ventilators. The main aim was to prevent deaths, hospitalisations, severe infections, moderate the symptoms, no symptoms. Probably not in that order, but no infection at all was never the main aim of any vaccines. The vaccines have been developed to boost immunity and to curb the number of hospital admission as well as to control the new cases.







